Pramod P Wangikar
Core Faculty
Professor

136, Chemical Engineering
Core Faculty
Professor
National Bioscience Award for Career Development 2006 (DBT Government of India).
INAE Young Engineer Award 2005
G. R. Manudhane Excellence in Research Award (IIT Bombay) 2005
BOYSCAST fellowship (DST Govt of India) 2003
DAE Young Scientist Award (Dept. of Atomic Energy Govt. of India) 1997.
AICTE Career Award for Young Teachers 1998.
Deployment of enzymes in industrial production of chemicals requires development on three fronts: (i) Design of enzymes with desired char- acteristics of activity, selectivity, stability, substrate tolerance, etc., (ii) Cost effective production of the enzyme and (iii) Development of bio-transformation process. Wangikar lab’s current efforts are on all three fronts with focus on developing processes for chiral synthesis using two classes of enzymes; nitrilase and alcohol dehydrogenase (ADH). De-sign of enzymes is initiated with experimental testing of unexplored sequences of putative enzymes followed by directed evolution of prom-ising candidates. We also undertake rational, model based design of en-zymes in collaboration with other groups that have expertise in protein X-raycrystallography. Currently, the group has designed novel ADH enzymes that show >99% stereoselectivity and > 100 units activity / ml of fermentation broth. We envisage the use of whole cell biocatalyst for cost effective chiral synthesis. We have also designed acofactor recycle system with a recycle ratio of 1:2,000.
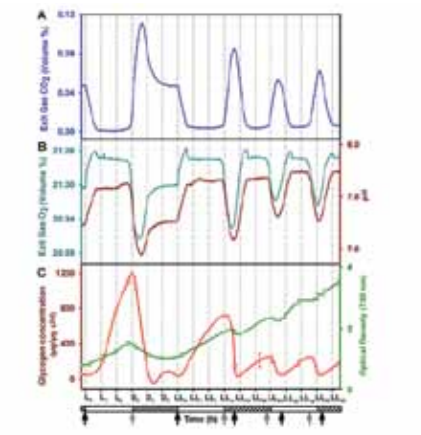
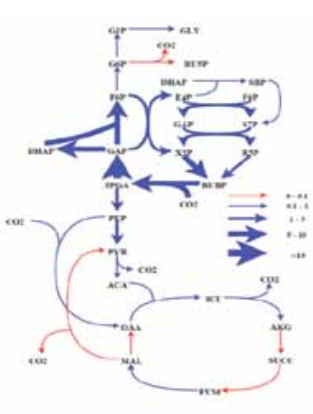
Atmospheric CO 2 levels have been rising at alarming rates over the last two centuries, and are projected to reach 700-1000 ppm (parts per mil-lion) by year 2100. Carbon and energy accounting reveals that a typical microalgal CCU is far from being net carbon negative or net energy positive, primarily because of high operational energy requirements. Further, even a medium sized Thermal Power Station would require algal ponds of over hundred square kilometers, thereby posing the challenge of capital and scale. Wangikar’s group works towards design of algal strains and processes to improve the aerial productivity. Their strain selection criteria include: (i) volumetric and aerial productivity, (ii) bio-mass concentration at the end of batch, (iii) tolerance to local climatic conditions, (iv) tolerance to CO 2 and other flue gas components, and (v) ability to synthesize storage molecules. Algal strains isolated from
the plant site of our industry partner fulfill many of these requirements and are now ready to be tested for CO 2 capture at the plant site. Apart from this, Wangikar’s group also works on metabolic engineering of cyanobacteria for photoautotrophic production of high value compounds. This includes physiological characterization, genome sequencing, metabolic model construction, flux analysis and several other systems biology approaches. We have performed 13C flux analysis on model strains of cyanobacteria with current efforts on local strains. Further, we have identified a number of cyanobacterial promoters that are under the control of an internal circadian clock.