102 Silicate Lab, IIT Bombay,
Powai, Mumbai – 400076

Education:
PhD (2021): IITB- Monash Research Academy (A dual-badged PhD degree from Indian Institute of Technology Bombay, India and Monash University, Australia).
Post Graduation (2015): Master of Biotechnology, All India Institute of Medical Sciences, New Delhi, India.
Graduation (2013): B.Sc.(H) Biochemistry, University of Delhi, Delhi, India.
Orcid ID: https://orcid.org/0000-0003-4600-3744
Thesis topic: Ophthalmic drug delivery with porous silicon
Research summary:
Eye is a self-protected fortress inside the human body. Its anatomical location and design render it almost impermeable. The ocular barriers are a tight regulator of passage of solutes and fluids inside the eye. Similarly, the permeability and bioavailability of drugs and biologicals in the eye is also impacted by the stringency of these barriers. Hence, despite the advances science has made till date, drug delivery to the eye remains a challenge.
Most of the currently available therapy for eye rely on topical administration of drugs as eye drops or ointments. A majority of therapeutic agent present in these formulations is ejected from the eye along with the tear soon after administration. The corneal permeability is typically very low, and therapeutic doses seldom reach the tissues at the back of the eye. Specialised administration routes are employed to delivery therapeutic drugs to the retina and other tissues at the back of the eye. These require repeated injections into the eye, causing distress to the patients. Owing to the blood-ocular barriers, despite such invasive procedure the drug bioavailability is poor and often not enough for therapeutic purposes.
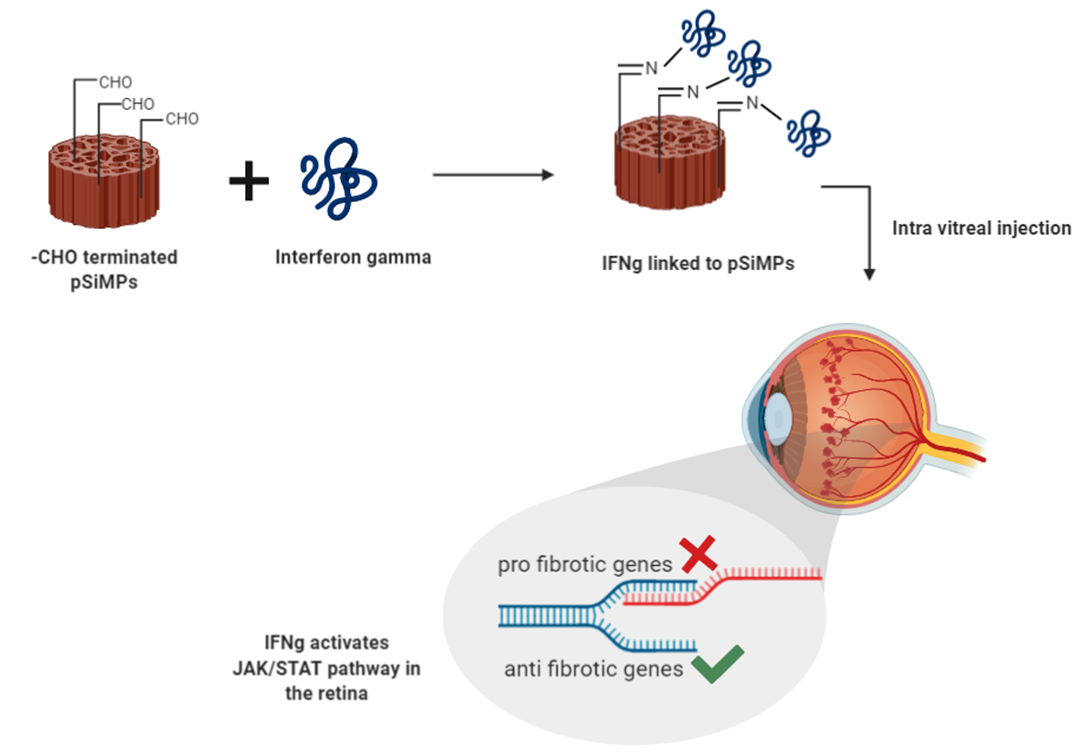
My current work addresses the urgent need to develop delivery systems for diseases that affect the posterior segment of the eye. I work with porous silicon based sustained-release micro-carriers which have an increased payload of therapeutic molecule. My work combines targeting novel pathways using newer carriers to overcome the traditional challenges associated with conventional therapies. Interferon gamma is an immune active cytokine that activates the JAK-STAT pathway. It is implicated to be involved in downregulating the modulators of fibrosis. Fibrosis is a key pathology associated with retinal damage in Age-Related Macular Degeneration (AMD). It, however, is not targeted for therapeutic purposes.
I use porous silicon microparticles prepared by standard etching method. These particles were then modified to have a free aldehyde group which forms an imine with the Interferon gamma, covalently linking the protein to the particle. We achieved up to 250 microgram Interferon gamma loading per milligram of porous silicon particles. These particles are capable of sustained release of Interferon gamma for up to 30 days. In vitro testing in ARPE 19 cells, an established model cell line for AMD studies has indicated the potential of porous silicon as a sustained release system for ophthalmic use. Further, in vivo studies are in progress to evaluate Interferon gamma loaded porous silicon particles for treatment of AMD.
Carriers like porous silicon overcome the traditional challenges in ocular drug delivery and improve patient care by reducing the need for repeated dosing and invasive procedures.
Keywords: Ocular, drug delivery, age-related macular degeneration, porous silicon, fibrosis, Interferon gamma, sustained drug release
Graphical representation of research work: