Madhu Vinjamur
Core Faculty
Professor

302, Chemical Engineering
Core Faculty
Professor
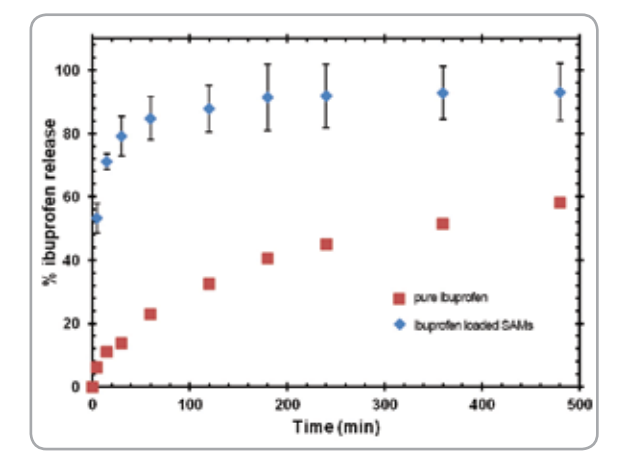
An improved process for preparation of silica aerogel microparticles (SAMs) for drug delivery from rice husk ash (RHA), an inexpensive source rich in biocompatible silica, has been developed. The wet gel microparticles were produced by a sol–gel method using water-in-oil emulsion, where a mineral oil replaced vegetable oil for easy separation using less energy. Taguchi design of experiments was used to optimize the parameters controlling the sol–gel method. The wet gel particles were dried with supercritical carbon dioxide (scCO2 ) to obtain SAMs. They were characterized by their properties such as BET surface area, pore volume, pore diameter and morphology. The efficacy of the improved process was validated by loading a water insoluble drug, ibuprofen, and a food preservative, eugenol, in SAMs from scCO2 medium. SAMs had a total porosity of 99.34%, surface area of 652 m2 /g and a pore volume of 3 cm3 /g. High loadings of 0.87 g ibuprofen/g of aerogel and 8.1 g eugenol/g of aerogel were obtained. Nearly 80% of the ibuprofen was released in 30 min from SAMs compared to 14% of ibuprofen sold in the market. High loading and fast release kinetics confirmed that SAMs produced by the process are suitable for drug delivery.
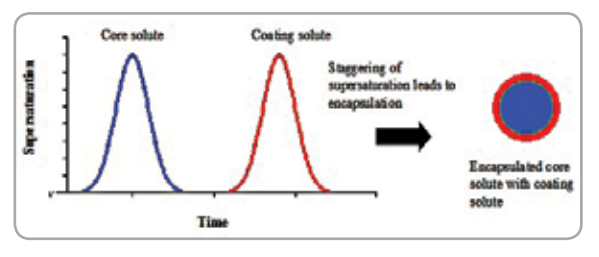
A rapid pressure reduction over a CO2 -expanded organic solution, from 30–70 bar to 1 bar, decreases the solution temperature by 30–80 K in a short span of time (0.5–1.5 min). This decrease generates a rapid, high, and uniform supersaturation of the dissolved solute in the solution and facilitates its precipitation as ultrafine particles. A model is developed to estimate the supersaturation, nucleation and growth rates during the pressure reduction and the particle size distribution of the precipitated particles. Cholesterol has been chosen as a model solute and acetone as a solvent. Equilibrium solubility of solute is affected by CO2 mole fraction and solution temperature during pressure reduction. Size distributions of the precipitated particles have been calculated assuming primary nucleation (homogeneous and heterogeneous nucleation) and diffusion-limited growth kinetics. The particle sizes predicted by heterogeneous nucleation are found to be closer to the measured sizes than homogeneous nucleation.